Working with Mel Science . Chemistry Sets
I started working with the Tin kit today at The Laboratory. Two guided experiments are available with this kit: 1) Tin Hedgehog (I immediately thought of Sonic the Hedgehog [1]) and 2) Tin Dendrites.
Tin (錫) [2] has a storied history in China, as an important component in bronze weapons, bells, and vessels. From the classic text 天工開物 comes these passages describing tin, also known as 賀 from ancient texts.
凡響銅入錫參和。成樂器者…
From 錘鍛第十.治銅
凡錫中國偏出西南郡邑,東北寡生。古書名錫為賀者。以臨賀郡產錫最盛而得名也。…凡錫有山鍚,水錫兩種。…
From 五金第十四.錫

The kit comes with an app that can be downloaded onto a smart phone. I found the Mel Chemistry app to be intuitive, although at times with gloves on (latex gloves come with the kit) I hesitated to press the touch screen and buttons. With my science background I could say that both experiments are safe provided sufficient ventilation in the working space, and I was impressed with the attention to safety Mel Science takes with its instruction manuals. There are largely labeled “Read the Instructions First for Safety” on the paper manuals which I dutifully followed, and on the first few pages there are blanks for filling in local poison control/hospital contact phone numbers (which I filled in before starting anything). This information is not to scare or deter in any way but is actually great information to have on hand always. Some more on safety, the kit also comes with laboratory type protective eye wear, which fits over my glasses.
I had to press the buttons and screens a few times to jump back and forth between the camera app and Mel Chemistry app. Thinking on it now, I probably could have just saran wrapped the entire phone for the experiment to allay any touchy fears. The instructions are clearly written on the app and paper manuals, though at several points of the experiment if there were instructions to stop, take photos or videos now then I would have captured on video my personal “Aha” moments. Perhaps for first time experimenters, those moments are priceless anyways.
The kits come with all the chemistry equipment needed (except AAA batteries) and I proceeded without any significant delay. One drawback on the paper manuals but more thoroughly done on the App is how to deal with disposals, and in both cases there should probably be more attention to instructing how to properly cleanup after experiments are completed.
Tin Hedgehog.
The experiment consists of preparing a SnCl_2 solution by mixing 10 mL of NaHSO_4 with SnCl_2 granular powder. I started by adding droplets of the sodium bisulfate into the tin(II) chloride, then after making sure nothing bad happens, squirted larger quantities into the powder. The resulting solution is rather milky and translucent and viscous. I poured the solution to fill a plastic vial halfway. An important observation from my perspective at this point is that the solution does not have a meniscus that is either convex or concave and is instead rather flat (at least visually to the eye) in the plastic vial, indicating a nearly 90 degree contact angle! A follow up measurement of interest would be to check the surface tension (something called the Wilhelmy plate [3]).

Then an ~2.6 g Zinc (Zn) pellet is dropped into the solution to initiate the following chemical reaction: SnCl_2 + Zn –> Sn + ZnCl_2. The spiky, flaky Sn pieces become evident after a few minutes; the kit instructions call for letting the reaction run for nearly 15 minutes or so. It would be interesting to analyze the Sn and Zn purity in some more detail (more on this later perhaps).

Interestingly, zinc has been called 倭鉛 or 亞鉛 [4] in one of the classic Chinese texts 天工開物. Here’s an excerpt of the passage describing Zinc.
凡倭鉛,古書本無之,乃近世所立名色。其質用爐甘石熬煉而成。繁產山西太行山一帶,而荊、衡為次之。每爐甘石十觔,裝載入一泥罐內,封裹泥固,以漸砑幹,勿使見火拆裂。然後,逐層用煤炭餅墊盛,其底鋪薪,發火煆紅,罐中爐甘石熔化成團。冷定,毀罐取出。每十耗去其二,即倭鉛也。此物無銅收伏,入火即成煙飛去。以其似鉛而性猛,故名之曰“倭”云。
From 五金第十四.附倭鉛
Tin Dendrite.

This next experiment is as visually stunning as the previous one. The prepared tin(II) dychloride is poured into a petri dish. The liquid has high surface tension on plastic and does not “wet” the petri dish completely. Mel Chemistry includes a soapy concoction in the kit — a single droplet of the soap immediately forces the Sn solution to run over and cover the entire petri dish surface area. This reminds me of what happens when working with HF and Silicon native oxide; after the oxide is etched [5] the HF will completely wet the silicon.
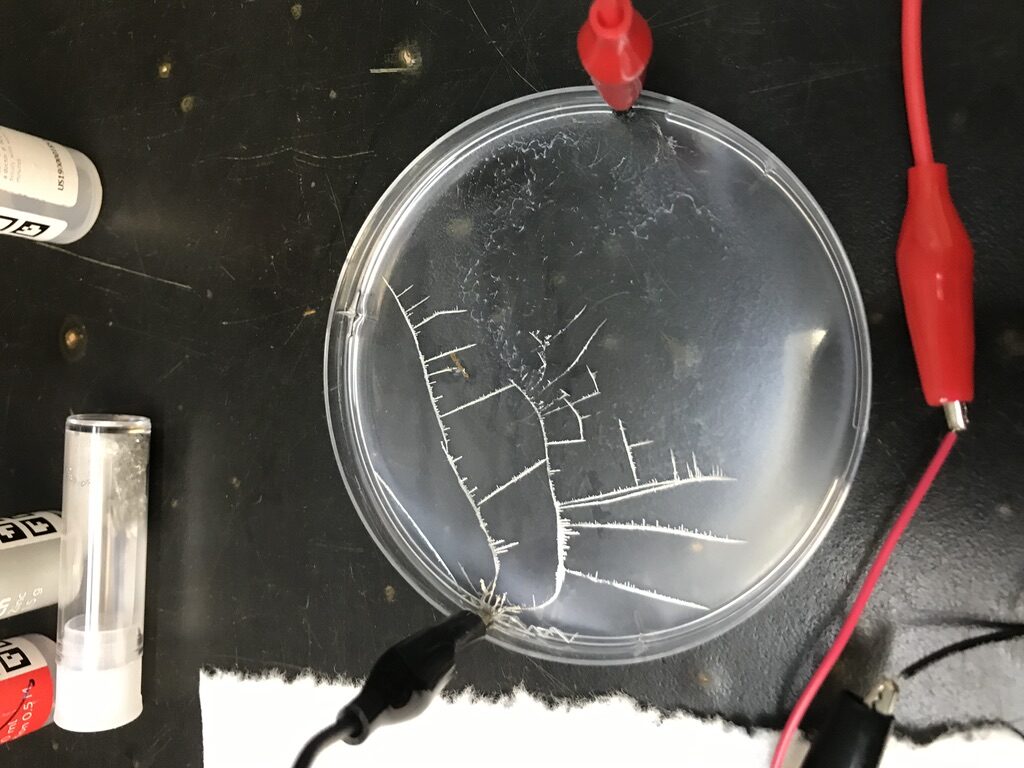
Once batteries are connected to the solution, dendritic Sn starts to form, creating a visually compelling image of electrochemistry in action. I noticed that the dendrite will form at either the positive or negative electrodes depending on how the circuit is connected. Contrasting to what is described in the manual, I didn’t see dendrites start to disappear when changing polarity (the dendrites are fragile and do disappear if I shake the petri dish with medium force).
The electrochemistry is aptly described with detail in the app. What happens is the metal Sn precipitates from the solution when driven by the applied electrical energy, Sn^(2+)_(solution) + 2e^- –> Sn_(solid). At the anode (the electrode the Sn tries to grow towards), water is dissociated 2H_2O + 4e^- –> O_2 + 4H^+. This reaction combined with the tin solution leads to 2SnCl_2 +O_2 +2H_2O –> 2SnO_2 + 4HCl occurring. This experiment is visually very beautiful because it shows the ion current in solution, spatially and temporally.
References
- https://en.wikipedia.org/wiki/Sonic_the_Hedgehog
- https://zh.wikipedia.org/wiki/锡
- https://en.wikipedia.org/wiki/Wilhelmy_plate
- https://zh.wikipedia.org/wiki/锌
- https://www.mdbg.net/chinese/dictionary?page=worddict&wdrst=1&wdqb=etch
在〈“Mel Science . Chemistry – Tin 錫”〉中有 1 則留言
留言功能已關閉。